
Invloed van apolaire staart op oplosbaarheid van alkanolen in water YouTube
The gas is made of two types of atoms - Carbon and Oxygen. Each CO2 molecule has two oxygen atoms and one carbon atom. If you study the CO2 Lewis structure and its molecular geometry, you will know that the Carbon atom is in the central position forming double bonds with both Oxygen atoms. This structure can help us understand the polarity of this molecule.

MkMolcules organiques
If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. We have Oxygen atoms on one side and a Hydrogen atom on the ot.

Chemie polair en apolair Olie azijn die elkaar eigenlijk afstoten. Deze vloeistoffen kan je
Learn to determine if PCl3 (Phosphorous trichloride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the.

HAVO Hfst2 opgave 29 polair apolair YouTube
Begrippen polair en apolair. Verschuiving van bindingselektronen. Polaire of apolaire binding. Symmetrische of asymmetrische moleculen. Polair of apolair molecule.

Apolair vs polair YouTube
Is BeCl2 Polar or Nonpolar? (Beryllium chloride)Learn to determine if BeCl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (sha.
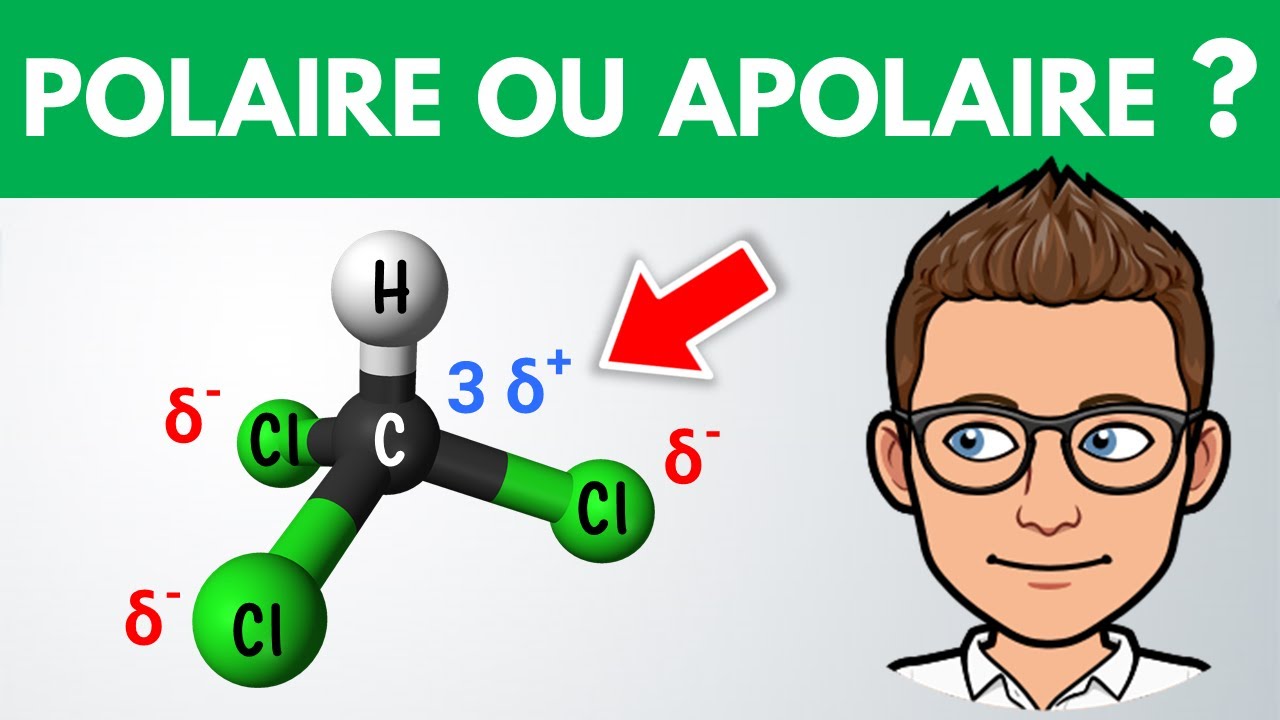
Molécule polaire ou apolaire ? ️ Exercice 1ère Physique Chimie YouTube
Learn to determine if BCl3 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us.
Huishoudelijke apparaten Silicagel polair of apolair
A solvent is simply a substance that can dissolve other molecules and compounds, which are known as solutes. A homogeneous mixture of solvent and solute is called a solution, and much of life's chemistry takes place in aqueous solutions, or solutions with water as the solvent. Because of its polarity and ability to form hydrogen bonds, water.
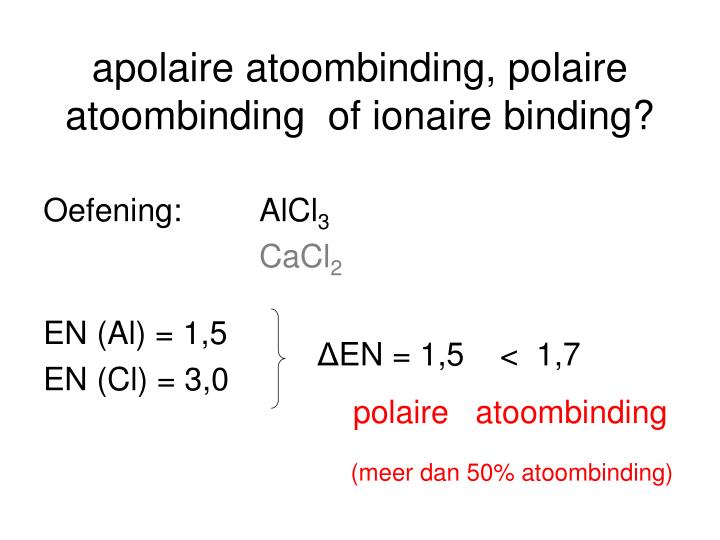
PPT 2.Fijnstructuur van moleculen 2.2 Soorten bindingsmodellen PowerPoint Presentation ID
Yes, caffeine is a polar molecule. The Oxygen and Nitrogen molecules have a stronger polarity than Carbon, allowing them to slightly pull the electrons towards them in their covalent bond. This will give those atoms a slightly negative charge while giving the Carbon a positive charge. This is the exact definition of polarity itself.

Synthese polair apolair YouTube
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright.

Polaire en apolaire moleculen Mr. Chadd Academy
Een polaire verbinding is een molecuul dat bestaat uit een chemische binding met een zodanige verdeling van de elektronen, dat het centrum van de negatieve ladingen niet samenvalt met dat van de positieve ladingen. Een polaire verbinding is het tegenovergestelde van een apolaire verbinding. Moleculen met zowel een polair als een apolair deel.

Chemie oplosbaarheid van stoffen (polair/apolair) YouTube
Sommige stoffen zijn polair, andere apolair. Een molecuul is polair als de elektronen (die een negatieve lading hebben) in dat molecuul dusdanig verdeeld zij.

Polair apolair + begin chemisch rekenen filmpje groter YouTube
Its chemical formula is CH3OH containing one carbon atom, one oxygen, and four hydrogen atoms. The molecular mass of methanol is 32.04 g per mol. It is calculated as. 1* (mol mass of C) + 4* (mol mass of H) + mol mass of O. 12+1×4+16 = 32 grams. Methanol does not have texture, it is a colorless liquid and smells like ethanol.

Polair & Apolair YouTube
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic properties and was formerly important as a topical antiseptic.The medical use of the dye has been largely superseded by more.
Apolair molecule — Chemieleerkracht
Yes, NH3 (Ammonia) molecule is polar in nature because of its asymmetrical shape ie; trigonal pyramidal structure, and the difference in electronegativities of N (3.04) and H (2.2). The charges over the nitrogen and hydrogen atoms are unequally distributed which results in a net dipole moment making NH3 (Ammonia) a polar molecule.
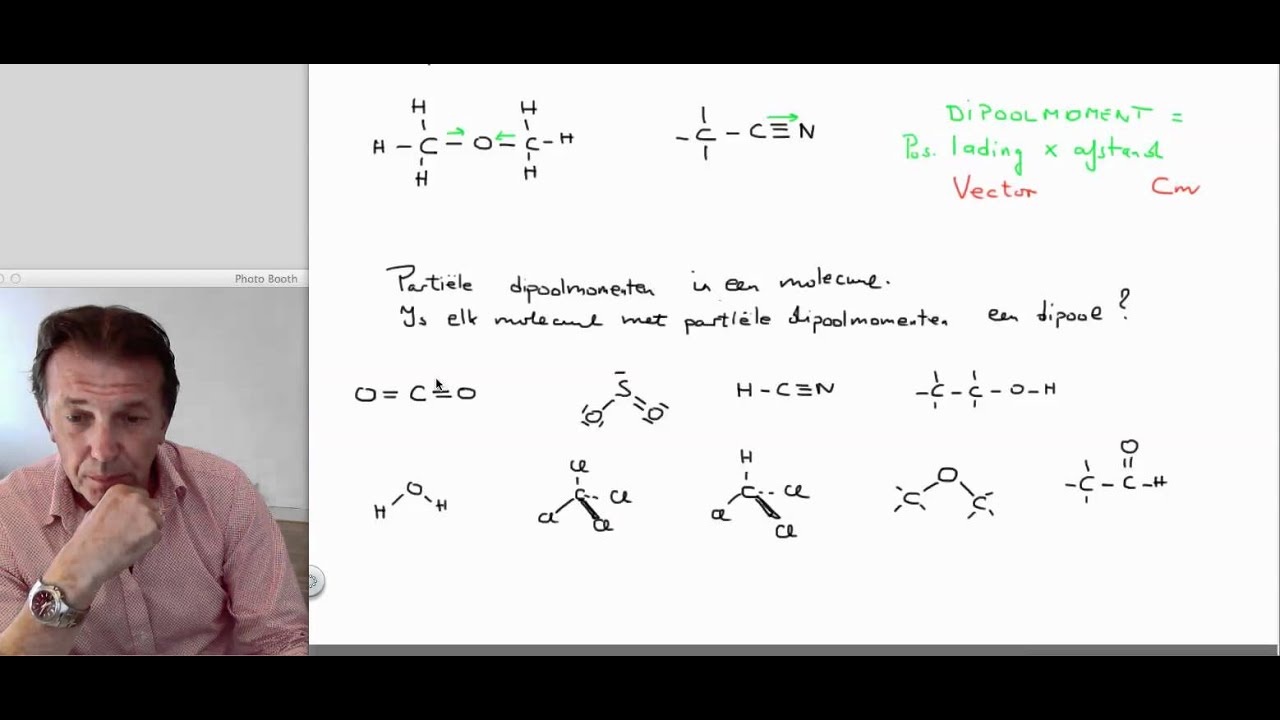
7 VWO Dipoolmomentdipolenpolairapolair scheikunde YouTube
It is acidic in nature and has an acidity of 9.21 PKA. The melting point of this substance is −13.29 °C or 8.08 °F, and its boiling point is 26 °C or 79 °F. At a temperature of 25 °C, its vapor pressure is 100 kPa. The polarity of HCN is 2.98 D. The molecular shape of HCN is linear.

Oplossingen polair of apolair YouTube
IUPAC Standard InChIKey: HZYHMHHBBBSGHB-ODYTWBPASA-N Copy CAS Registry Number: 557-48-2 Chemical structure: This structure is also available as a 2d Mol file.